What Does It Means to Be a Group or Family in Te Periodic Tablle
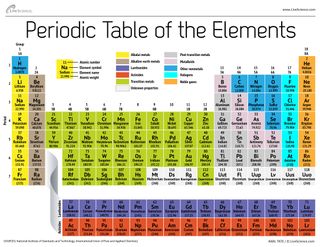
How the Periodic Table of the Elements is arranged
Scientists had a rudimentary agreement of the periodic table of the elements centuries ago. But in the late 19th century, Russian pharmacist Dmitri Mendeleev published his starting time try at grouping chemical elements according to their diminutive weights. There were only about 60 elements known at the fourth dimension, merely Mendeleev realized that when the elements were organized past weight, certain types of elements occurred in regular intervals, or periods.
Today, 150 years later, chemists officially recognize 118 elements (after the addition of 4 newcomers in 2016) and still utilize Mendeleev's periodic tabular array of elements to organize them. The table starts with the simplest atom, hydrogen, and and so organizes the rest of the elements by atomic number, which is the number of protons each contains. With a scattering of exceptions, the order of the elements corresponds with the increasing mass of each atom.
The table has seven rows and 18 columns. Each row represents one period; the period number of an element indicates how many of its energy levels house electrons. Sodium, for instance, sits in the tertiary period, which means a sodium atom typically has electrons in the starting time three free energy levels. Moving down the table, periods are longer because it takes more electrons to make full the larger and more complex outer levels.
The columns of the table represent groups, or families, of elements. The elements in a group often expect and behave similarly, considering they take the same number of electrons in their outermost shell — the confront they show to the world. Grouping 18 elements, on the far right side of the tabular array, for example, accept completely full outer shells and rarely participate in chemic reactions.
Elements are typically classified every bit either a metal or nonmetal, but the dividing line between the two is fuzzy. Metal elements are usually good conductors of electricity and heat. The subgroups within the metals are based on the similar characteristics and chemical properties of these collections. Our description of the periodic table uses unremarkably accepted groupings of elements, according to the Los Alamos National Laboratory.
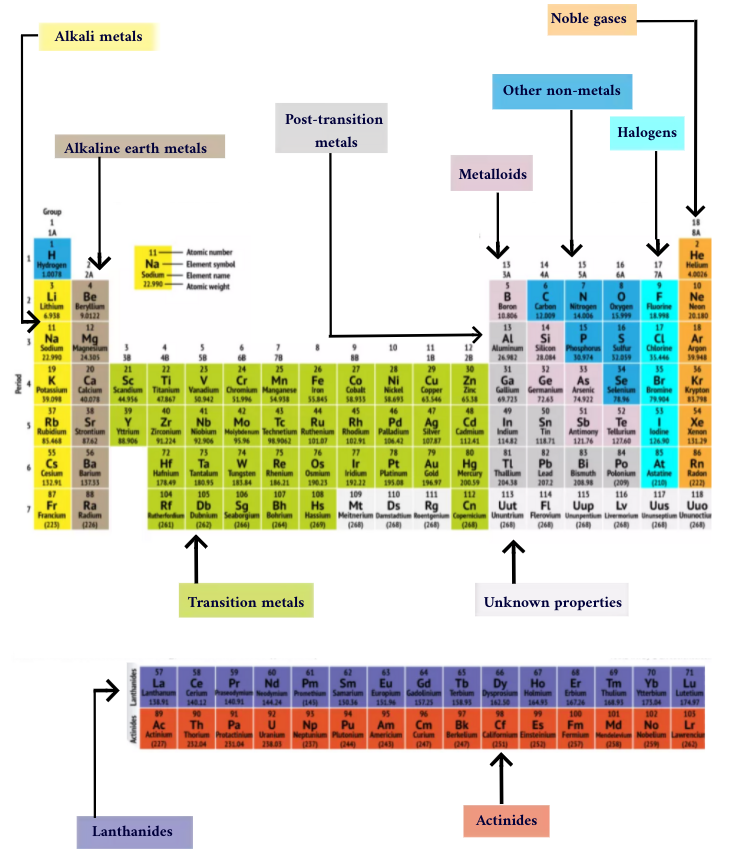
Groups of the Periodic table
Brine metals: The alkali metals make up most of Grouping 1, the table'southward kickoff column. Shiny and soft plenty to cut with a pocketknife, these metals start with lithium (Li) and end with francium (Fr). They are also extremely reactive and will burst into flame or fifty-fifty explode on contact with water, and then chemists store them in oils or inert gases. Hydrogen, with its single electron, besides lives in Group 1, but the gas is considered a nonmetal.
Alkaline-earth metals: The alkaline-earth metals make up Grouping 2 of the periodic table, from glucinium (Be) through radium (Ra). Each of these elements has 2 electrons in its outermost energy level, which makes the alkaline earths reactive plenty that they're rarely constitute solitary in nature. But they're not as reactive equally the alkali metals. Their chemical reactions typically occur more slowly and produce less heat compared to the alkali metals.
Lanthanides: The tertiary group is much likewise long to fit into the third cavalcade, so it is broken out and flipped sideways to become the elevation row of the isle that floats at the bottom of the table. This is the lanthanides, elements 57 through 71 — lanthanum (La) to lutetium (Lu). The elements in this group have a silvery white color and tarnish on contact with air.
Actinides: The actinides line the lesser row of the isle and contain elements 89, actinium (Ac), through 103, lawrencium (Lr). Of these elements, only thorium (Thursday) and uranium (U) occur naturally on Earth in substantial amounts. All are radioactive. The actinides and the lanthanides together form a grouping called the inner transition metals.
Transition metals: Returning to the main trunk of the table, the residual of Groups three through 12 stand for the rest of the transition metals. Hard but malleable, shiny, and possessing good conductivity, these elements are what yous typically think of when you lot hear the word metal. Many of the greatest hits of the metal globe — including aureate, argent, iron and platinum — live here.
Post-transition metals: Ahead of the spring into the nonmetal world, shared characteristics aren't neatly divided along vertical grouping lines. The mail service-transition metals are aluminum (Al), gallium (Ga), indium (In), thallium (Tl), can (Sn), pb (Atomic number 82) and bismuth (Bi), and they span Grouping xiii to Group 17. These elements accept some of the classic characteristics of the transition metals, but they tend to be softer and conduct more poorly than other transition metals. Many periodic tables will characteristic a bolded "staircase" line beneath the diagonal connecting boron with astatine. The post-transition metals cluster to the lower left of this line.
Metalloids: The metalloids are boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te) and polonium (Po). They form the staircase that represents the gradual transition from metals to nonmetals. These elements sometimes carry equally semiconductors (B, Si, Ge) rather than as conductors. Metalloids are too chosen "semimetals" or "poor metals."
Nonmetals: Everything else to the upper right of the staircase — plus hydrogen (H), stranded style back in Grouping 1 — is a nonmetal. These include carbon (C), nitrogen (Due north), phosphorus (P), oxygen (O), sulfur (S) and selenium (Se).
Halogens: The pinnacle 4 elements of Group 17, from fluorine (F) through astatine (At), represent ane of two subsets of the nonmetals. The halogens are quite chemically reactive and tend to pair upward with brine metals to produce various types of salt. The tabular array salt in your kitchen, for example, is a marriage betwixt the brine metal sodium and the halogen chlorine.
Noble gases: Colorless, odorless and near completely nonreactive, the inert, or noble gases round out the table in Group 18. Many chemists look oganesson (previously designated "ununoctium"), one of the four newly named elements, to share these characteristics; notwithstanding, because this element has a half-life measuring in the milliseconds, no ane has been able to test it straight. Oganesson completes the 7th menstruation of the periodic tabular array, so if anyone manages to synthesize element 119 (and the race to do so is already underway), it will loop around to kickoff row eight in the alkali metal column.
Because of the cyclical nature created past the periodicity that gives the tabular array its proper name, some chemists prefer to visualize Mendeleev's table as a circle.
Additional resources:
- Watch this brief video well-nigh the periodic table and chemical element groups, from Crash Course.
- Flip through this interactive periodic tabular array of elements at ptable.com.
- Check out this gratuitous, online educational resource for understanding elemental groups from CK-12.
Source: https://www.livescience.com/28507-element-groups.html
0 Response to "What Does It Means to Be a Group or Family in Te Periodic Tablle"
Post a Comment